George Cove, a forgotten solar power pioneer, may have built a highly efficient photovoltaic panel 40 years before Bell Labs engineers invented silicon cells. If proven to work, his design could lead to less complex and more sustainable solar panels.
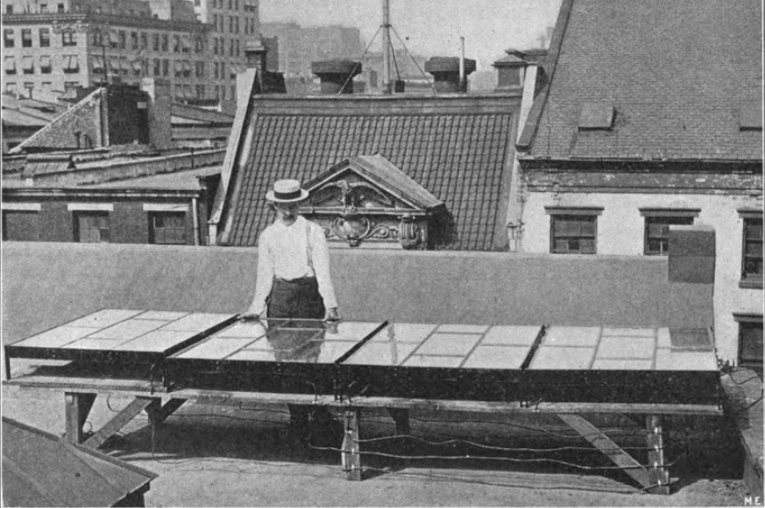
Above: George Cove stands next to his third solar array. Source: “Generating electricity by the sun’s rays”, Popular Electricity, Volume 2, nr. 12, April 1910, pp.793.
More efficient, less sustainable
Ever since Bell Labs presented the first practical solar PV panel in the 1950s, technological development has focused on reducing costs and increasing the efficiency of solar cells. According to these standards, researchers have made a lot of progress. The efficiency of solar panels increased from less than 5% in the 1950s to over 20% today, while the costs decreased from 30 dollars per watt-peak in 1980 to less than 0.2 dollars per watt-peak in 2020. Lower costs – to which higher efficiencies contribute – are considered of paramount importance because they allow solar PV panels to compete in the market with electricity generated by fossil fuels.
However, in terms of sustainability, very little progress has been made. To start with, ever since the 1950s, solar panels have been unfit for recycling, resulting in a waste stream that ends up in landfills. This waste stream will grow significantly during the coming years. Solar panels are discarded only after at least 25 to 30 years, and most have been installed only in recent years. By 2050, researchers expect that almost 80 million tonnes of solar panels will reach the end of their lives. [1-3] That is a significant waste of resources and a danger to the environment – discarded solar PV panels contain toxic elements and present a fire hazard.
The manufacturing of solar PV panels is just as problematic. It produces toxic waste and requires a global supply chain, including capital-intensive factories, complex machinery, mined materials, and a steady input of fossil fuels. In life cycle analyses of solar panels, scientists calculate how much energy and materials are required to build a solar panel. However, they ignore the massive amount of energy and materials needed to set up and maintain the solar PV supply chain itself. [4-11] Consequently, these studies do not reveal the actual cost of solar panels in terms of fossil fuel dependence, emissions, and other environmental pollution. Furthermore, the need for capital-intensive technology and long supply lines prevents the local production of solar panels by less affluent societies or DIY communities.
Finding inspiration in the past
Are solar PV panels inherently unsustainable, unrecyclable, and dependent on high-tech and capital-intensive manufacturing processes? Or, is it possible to build them with local, recyclable and less energy-intensive materials and production methods? In other words, can we build low-tech solar panels? And, if so, what would that mean for costs and efficiency?
Before we try to answer this question, it’s important to note that the best low-tech alternative for a high-tech solar panel is often not a low-tech solar panel but direct use of solar energy. That is, putting solar energy to use without converting it to electricity first. For example, a clothesline and a solar thermal water boiler are much more efficient, sustainable, and economical than an electric tumble dryer and a water boiler powered by solar PV panels. Direct use of solar energy can happen with local materials, relatively simple manufacturing technologies, and short supply lines.
Nevertheless, in this article, I take the question literally: can we build low-tech photovoltaic devices, which convert sunlight into electricity? In a previous article, we have seen that history offers inspiration for building more sustainable wind turbines. Can history also inspire us to make more sustainable solar cells?
The prehistory of solar cells
Bell Labs’ solar PV panel, presented in 1954, came not out of nowhere. The silicon solar cell had its roots in less complex devices that could produce electricity from either light or heat.
In 1821, Thomas Seebeck found that an electrical current will flow in a circuit made from two dissimilar metals, with the junctions at different temperatures. This “thermoelectric effect” formed the basis for the “thermoelectric generator” — which converts heat (for example, from a wood stove) directly into electricity. In 1839, Antoine Becquerel discovered that light could also convert into electricity, and during the 1870s, several scientists proved this effect in solids, most notably in selenium. This “photoelectric effect” formed the basis for the “photoelectric generator” — which we now call a “photovoltaic” generator or solar PV cell. In 1883, Charles Fritts constructed the first photovoltaic module ever made, using selenium on a thin layer of gold. [12-14]
Throughout this period – and until the 1950s – the practical uses of thermoelectric and photoelectric devices were limited. Inventors built many experimental thermoelectric generators, usually powered by a gas flame, but their efficiency did not exceed 1%. Likewise, Charles Fritts’ solar panel, and the selenium solar cells made afterward, obtained just 1-2% efficiency in converting sunlight into electricity. [15] In short, the period before the 1950s doesn’t seem to offer much inspiration for building more sustainable solar PV panels.
A forgotten pioneer of solar power
However, the prehistory of the solar panel may be incomplete. In 2019, I received a mail from a reader of Low-tech Magazine, Philip Pesavento:
“I have been studying an early pioneer in solar cell technology from the pre-WWI era since the early 1990s. I am getting too old to continue doing anything with this, and even though there have been one or two scholarly articles about Mr. Cove, they have completely missed what he accomplished. I am enclosing a PDF of a PowerPoint that I put together back in 2015 and never presented to anyone. If you are interested in pursuing writing a paper yourself, I could mail you a thumb drive with all the background material that I have collected.”
If Philip Pesavento’s historical account and hypotheses are correct, George Cove set out to build a thermoelectric generator but accidentally made a photovoltaic generator – a PV solar cell. Although this happened in the early 1900s, Cove obtained a comparable power output and efficiency to the Bell Labs scientists in 1954. His design also showed much higher performance than the selenium solar cells built between the 1880s and the 1940s. [16] Philip Pesavento:
“It would be quite exciting to prove that relatively high-efficiency solar cells were invented 40 years before the development of silicon cells. More importantly, if it turns out there was a solar photovoltaic cell and panel system before World War I, it might also have some advantages concerning the cheapness of raw materials, low embodied energy to convert the ores into metallic materials, the efficiency of the final PV cells, and ease of fabrication.”
In other words, if Philip Pesavento’s historical account and hypotheses are correct, it may be possible to build low-tech solar panels.
George Cove’s solar electric generator
George Cove presented his first “solar electric generator” in 1905 in the Metropole Building in Halifax, Nova Scotia, Canada. Apart from an image, there are no data about this panel. [17] However, its power output and efficiency were remarkable enough for US investors to send an expert to Halifax. Based on this expert’s examination of the machine, they then brought Cove to the US (Sommerville, Mass.) to continue the development of his device.
Cove presented his second solar electric generator there in 1909. This 1.5m2 panel could produce 45 watts of power and was 2.75% efficient in converting solar energy into electricity. By mid-1909, Cove had moved to New York City, where he presented his third prototype, a solar array consisting of four solar panels of 60 watt-peak each, which charged a total of five lead-acid batteries. The total surface area was 4.5 m2, the maximum power output was 240 watts, and efficiency rose to 5% – similar to the first solar panel presented by Bell Labs. [18]
Above: George Cove’s first solar panel, demonstrated in 1905. Source: Technical World Magazine 11, nr.4, June 1909.
Above: Cove’s second solar panel, with one section missing. Source: Technical World Magazine 11, nr.4, June 1909.
Above: George Cove’s third solar panel. Source: “Harnessing sunlight”, René Homer, Modern Electrics, Vol. II, No.6, September 1909.
Above: George Cove’s third solar panel. The panels are now tilted at an angle as opposed to lying flat. Source: Literary Digest 1909.
Above: One of the solar panels of Cove’s third solar array, with the glass cover removed. Source: “Harnessing sunlight”, René Homer, Modern Electrics, Vol. II, No.6, September 1909.
Although George Cove is absent from most historical accounts of solar power, his solar electric generator impressed some popular tech media of the day. For example, in 1909, Technical World Magazine wrote that “such a machine is cheap and indestructible as a kitchen range. Even in its present and somewhat crude and experimental state, given two days of sun, it will store sufficient electrical energy to light an ordinary house for a week. The inventor has proved this now for months in his establishment”. [19]
Plugs set in asphalt
How did George Cove manage to build a solar panel that was 40 years ahead of its time? According to Philip Pesavento, who has a background in semiconductor engineering, Cove intended to build a better thermoelectric generator (TEG). He exposed his generator to the heat from a wood stove and direct solar energy — Edward Weston had made the first experimental solar thermoelectric generator (or STEG) in 1888. Cove’s intentions are also clear from how he described his device:
“The frame contains a number of panes of violet glass, behind which are set, through an asphalt compound backing, many little metal plugs. One end of the plugs is always exposed by sunlight, while the other end is cool and sheltered.”
Creating the largest possible temperature difference is key to thermoelectric power production, so Cove’s design makes sense. The problem is that when he measured the power output of his generator, it did not respond to heat like a thermo-electric generator was supposed to do. Initially, Cove observes that his invention uses both heat and light to produce electricity when exposed to solar energy:
“The principal part of my invention is the peculiar composition of the metallic plugs which are acted upon by the sun in such a way that the current is generated not only by heat rays but the violet rays as well”.
However, after further experiments with both the wood stove and solar energy, Cove states:
“When the machine is exposed to various sources of artificial heat it gives no electricity whatsoever. Other than the heat rays of the sun (short-wave infrared), perhaps the violet or ultraviolet rays are active in setting up the electrical current”.
The primary cell of Cove’s solar PV panel was a three-inch-long plug or rod of metallic composition, an alloy of several common metals. The 1.5 m2 panel had 976 rods, while the 4.5 m2 array had 4 x 1804 plugs. However, keeping the rods cool on one side and hot on another – separated by an asphalt layer – did not matter. What mattered is that Cove had unknowingly built a metal-semiconductor contact.
The semiconductor bandgap
George Cove did not understand how his solar generator worked, and neither did anyone else at the time. It was only with Einstein’s work on the photoelectric effect (in 1905) and later work in quantum mechanics (1930s and beyond) that the concept of a *semiconductor bandgap* was realized. Electrons orbit the nucleus of an atom in different “states”, which form regions that are called “bands”. These bands keep their electrons firmly in place. In between these bands are “bandgaps” – states in which no electron can be.
Conductors have no bandgaps, and so electrons flow through them. That is why a copper wire conducts electricity, for example. In insulators (like wood, glass, plastics, or ceramics), there is a very wide bandgap, which blocks the flow of electricity. Finally, in semiconductors, there’s a relatively narrow bandgap. That allows them to either act as an insulator or a conductor. Semiconductors can become conductors when they absorb a “photon” (an elementary particle of light) with an energy potential equal to or greater than the bandgap of the semiconductor material. [20]
The understanding of semiconductors led to the birth of the modern solar PV cell in the 1950s. It also improved the performance of thermoelectric generators – be it for different reasons. Thermoelectric generators do not take advantage of the semiconductor bandgap. However, semiconductors have higher thermo-voltages and lower thermal conductivities than metal and metal alloys with no bandgap, making thermoelectric generators more efficient.
The Schottky Junction
For a photovoltaic effect to exist, there must be some inhomogeneity in the system. In the 1950s, Bell Labs scientists managed to do this with the so-called p-n junction, which forms a boundary between a positively charged and a negatively charged semiconductor. P-type semiconductors have electron vacancies called “holes” (which attract electrons), while N-type semiconductors have extra electrons. At the junction between both is an electric potential.
However, it’s also possible to create a PV cell from a so-called Schottky junction, which connects a semiconductor with a metal. In this case, the metal functions as the n-type semiconductor. Philip Pesavento:
“My hypothesis is that George Cove stumbled upon a Schottky contact photovoltaic cell, decades before it was described by Walter Schottky. [21] There is the possibility of both photovoltaic (predominantly) and thermoelectric responses from these devices. The plug was an alloy of zinc and antimony – which we now know is a semiconductor. It was alternately capped by German silver (a nickel, copper, and zinc alloy) and copper on opposite ends. This formed an ohmic contact and Schottky contact, respectively. This is a photovoltaic device.”
Accidental discovery
According to Philip Pesavento, George Cove probably started with “German silver” as the negative material on both ends of the plugs, and an antimony-zinc alloy (ZnSb) as the positive material. These were the best available thermoelectric materials at the time:
“He probably ran out of German silver and substituted copper to finish making up a bunch of plugs since the difference in thermoelectric voltage between using copper and German silver was small. Then, during testing, Cove noted that these plugs (with a German silver cap at one end and a copper cap at the other end) gave a much greater voltage: 100s of mV’s versus the usual 10s of mV for a thermoelectric generator.”
What happened? By using copper, Cove had unknowingly built a Schottky junction. That converted his thermoelectric generator into a “thermophotovoltaic generator.” Such a device works the same as a photovoltaic solar cell but on a different wavelength. The solar spectrum covers a range of approximately 0.5 to 2.9 electron-Volts (eV), from infrared to ultraviolet. A semiconductor with a bandgap between 1 and 1.7 eV efficiently converts visible light into electricity (a photovoltaic generator) — while a semiconductor with a bandgap between 0.4 and 0.7 eV efficiently converts short-wave infrared solar energy into electricity (a thermophotovoltaic generator).
Above: This drawing from Cove’s 1906 patent shows the zinc-antimony alloy “b”; the german silver (ohmic) end cap “c”; and the copper or tin (Schottky) end cap “f”. All these are press-fit because soldering the connections lowered the efficiency.
We now know that ZnSb – the negative material in Cove’s plugs – is a semiconductor with a bandgap of 0.5 eV. That largely explains why the inventor initially observed that his solar generator converted both heat and light into electricity. A thermophotovoltaic generator matches not only the infrared tail of the solar spectrum — it also matches the direct spectrum of a burning flame or a red hot emitting surface which is heated by burning wood or natural gas. It also converts the lower portion of the visible spectrum into electricity, be it very inefficiently.
According to Philip Pesavento, Cove then managed to refine the composition of the alloy close to Zn4Sb3 – a zinc-antimony alloy with proportions of 4 parts zinc to 6 parts antimony. That, we now know, is also a semiconductor. However, it has a bandgap of 1.2 eV – very close to the bandgap of silicon (1.1 eV). Consequently, it turned his thermophotovoltaic generator into a photovoltaic generator:
“In his enthusiasm, Cove probably made up a larger number of plugs and somehow got the proportions “wrong” on one batch. He then measured an even larger voltage. Finally, he made a careful study of zinc-antimony alloys and found that the 40-42% range zinc alloy gave the highest voltage (compared to 35% zinc in ZnSb). Having – accidentally – discovered Zn4Sb3, the higher bandgap of this semiconductor meant that it no longer worked when it was exposed to the heat from a wood stove. However, it worked even better when it was exposed to solar energy – because it was now converting far more of the visible spectrum of sunlight efficiently into electricity.”
Using colored glass filters, George Cove determined that most of the response was from the violet end of the spectrum and only a little from the so-called heat rays. His earlier PV plugs had responded equally well to heat rays and violet rays, while the older thermoelectric generators (German silver at both sides) did not respond to the violet rays at all.
Bring back the Schottky solar cell?
Schottky junction solar cells have commanded only a small amount of attention from researchers and corporations – few solar cell designs use metals in the active region, other than for contacts. [22] Nevertheless, Philip Pesavento believes that it would be worthwhile to attempt to fabricate some Schottky solar cells according to Cove’s design:
“If it could be demonstrated that Zn4Sb3 (bandgap 1.2 eV) can be used in a photovoltaic cell, there is a good chance that such a solar cell design will be sustainable. It would be a good candidate for a quick EROI and have an acceptably long operational life with a surplus energy output over several decades. It’s astounding that everyone seems to have missed this material and its application to photovoltaic cells and that no development has been done – even after researchers briefly recognized it as being a possible option in the early to mid-1980s. It fits in the category of a premature discovery which should mean it could be developed very quickly in this day and age.”
Apart from solar PV, Philip Pesavento sees potential in thermophotovoltaics for a wood stove, solar thermal, or dual junction tandem applications, using ZnSb instead of Zn4Sb3. Furthermore, if the plug-type solar cells prove to be effective, he believes that they would allow line concentrator solar collectors – such as parabolic troughs or non-imagining CPC concentrators – to be built at greatly reduced costs.
Low-tech manufacturing
The primary advantage of Cove’s design would be its low-tech fabrication method. In the 1970s and 1980s, scientists investigated Zn4Sb3 for use in photovoltaics and concluded that the material’s “obvious advantages are apparent simplicity and relatively low temperature of the preparation procedure.” [23] The melting point for Zn4Sb3 is 570 degrees Celsius, while it’s 1,400 degrees for silicon.
Researchers studied metal-semiconductor junction solar cells based on other types of semiconductors than Zn4Sb3 in the 1970s. Again, their motivation was the simple and cost-effective fabrication procedure compared to silicon p-n junction solar cells at the time. [24, 25] Schottky cells do not require a high-temperature phosphorus-diffusion step, which ordinarily creates the n-layer of the p-n junction in silicon today. This alone reduces the energy input into the solar cell production process by 35%. [22]
During the 1980s, researchers made important advances in silicon p-n junctions, and interest in alternative configurations waned. However, there has been renewed interest in recent years. For example, research into graphene/silicon Schottky solar cells concludes that “simple and cost-effective device fabrication that does not require high temperatures is one of the advantages.” [26] In other recent studies, scientists conclude that Schottky-type “selenium devices are… extremely simple and cheap to fabricate”. [27-30]
Easier recycling
Another advantage of Schottky solar cells may be easier recycling. Silicon modules are sandwiched between two laminate encapsulant layers (usually EVA, an ethylene/vinyl acetate copolymer). These layers are essential to ensure module service lifetime. [1-3] To recycle the silicon – the most valuable component of a solar panel – these layers need to be separated, but burning them also destroys the modules. Silicon cells can only be recycled by a combination of thermal, chemical, and metallurgical steps. That is an expensive process with an impact on the environment. Although you can find statements claiming that around 10% of solar panels are “recycled”, they are more likely to be “downcycled”. The modules are shredded, and the resulting material is used as a filler material in asphalt and cement industries.
In contrast, the solar cells built by George Cove were entirely recyclable. They required no protective layer and did not even contain solder. Philip Pesavento:
“If you were to build the cells exactly the way Cove did by press-fitting the caps and then overwrapping them with wire to try to keep them tight, they would also be easier to recycle, being strictly a mechanical operation, no chemicals need to be involved. It would be labor-intensive to put them together and take them apart again, but it could be automated, too.”
Pesavento believes that it’s also possible to build flat solar cells from Cove’s material. However, whether or not those would need a protective layer that interferes with recycling remains to be seen. In the 1970s, Schottky solar cells based on other materials did not always need protective layers to reach more than 20 years of life expectancy. [24]
Efficiency
If we could build more low-tech solar panels, how efficient could we make them? According to Philip Pesavento, Schottky cells are slightly less efficient for the same materials than p-n junctions because p-n junctions generate a higher voltage – they get more of the energy in the photons they absorb.
“When every bit of efficiency counts, you do that. If making solar cells easier to manufacture using manual or artisan methods is your goal, the Schottky diode would be a more logical choice.”
On the other hand, it may be possible to build Schottky cells thinner than silicon solar cells – and that would increase their efficiency. Philip Pesavento:
“I have not found the specific numbers for the parameters – carrier velocity, recombination lifetime, absorption coefficient – to say this unequivocally. But the fact that Cove made such long skinny cells and got as high efficiencies as he did bodes well for making them thinner.”
Again, recent research into Schottky cells based on other materials seems to confirm this. For example, recent experiments with Schottky selenium cells brought layer thickness back to only 100 µm, compared to between 200 and 500 µm for silicon cells. [27] [31] Scientists also reached 17% experimental efficiency for a graphene/silicon Schottky cell, up from 1.5% ten years earlier. [26]
We can also question the current obsession with higher efficiencies. Many people will argue that if low-tech solar panels are less efficient, we would need more solar panels to produce the same power output. Consequently, the resources saved by low-tech production methods would be compensated by the extra resources to build more solar panels. However, efficiency is only crucial when we take energy demand for granted. A decrease in efficiency may just as well be compensated by lowering energy demand, especially when it leads to more sustainability and lower resource use throughout the supply chain. As with wind turbines, sacrificing some efficiency may gain us a lot in sustainability.
What happened to George Cove?
If Cove’s solar panel was so revolutionary, why is it forgotten? On this question, Philip Pesavento’s research material reads like a crime novel. Cove’s attempt to produce and market his solar energy device failed in mysterious ways.
The inventor became involved with a stock manipulator – Elmer Burlingame – who in 1909 and 1910 issued stock from businesses that were not his, including Cove’s start-up the Sun Electric Generator Company. In October 1909, Cove was allegedly kidnapped, and his life was threatened if he did not cease the development of his solar invention. However, the police dismissed Cove’s kidnapping as a hoax. In 1911, both Cove and Burlingame were arrested for stock fraud and spent a year in jail. Although Cove worked on other inventions after that, none of those were related to solar energy. [32]
Was George Cove a charlatan? Was he the victim of one? Or was his reputation destroyed because the solar electric generator threatened other companies’ interests? There are many historical examples of suppression of technological innovations by large US corporations. George Cove was active in the same period as the Edison Electric Illuminating Company of New York, whose unscrupulous practices against competitors are well-documented. If Cove’s solar electric generator worked, it could have reduced the growing demand for Edison’s coal and oil-fired power stations. [32] Earlier, in the 1880s, Edison had bought the company that produced the best thermoelectric generator at the time – Clamonds’s Improved Thermopile – and subsequently stopped the development of the machines. [33]
More Mysteries
However, while it’s tempting to see George Cove as a victim, we can only speculate. Philip Pesavento’s archive material contains more mysteries, such as Cove’s patent – applied for in 1905, granted in 1906. In his patent, the inventor describes the making of his Zn4Sb3 plugs in detail, which helped Pesavento to calculate the power output and efficiency of the solar arrays. However, Cove describes these plugs for converting heat from a wood stove into electricity, which is not compatible with his choice of material. To make the stove generator work, it required ZnSb plugs with a bandgap of 0.5 eV. Philip Pesavento:
“Was this misdirection on the part of Cove to prevent folks from copying his stove patent and getting it to work? I don’t know.”
Even more surprisingly, an image that shows Cove standing beside one of his solar panels also appears in John Perlin’s 2013 historical overview of solar power *Let It Shine: The 6,000-Year Story of Solar Energy*. However, the solar panel in the image is attributed to Charles Fritts, the inventor of the selenium solar cell. Furthermore, George Cove himself has disappeared from the image. Excerpts from the book, as well as the photo, have appeared on several websites. Philip Pesavento was not surprised when I got back in touch:
“I made this discovery several years ago. I guess that somebody badly needed an image of Fritts’ solar panels, found this image, and then photoshopped George Cove out of it. After all, Cove is totally unknown and when known is thought to have invented a solar thermoelectric generator, not a solar PV panel. If you look closely at the two photos, you can see that the top of the right column portico behind him was cut and pasted to where Cove had been standing, it’s not quite right in its perspective.”
Kris De Decker
References
[1]: Weckend, Stephanie, Andreas Wade, and Garvin A. Heath. End of life management: solar photovoltaic panels. No. NREL/TP-6A20-73852. National Renewable Energy Lab.(NREL), Golden, CO (United States), 2016.
[2]: Xu, Yan, et al. “Global status of recycling waste solar panels: A review.” Waste Management 75 (2018): 450-458.
[3]: Sica, Daniela, et al. “Management of end-of-life photovoltaic panels as a step towards a circular economy.” Renewable and Sustainable Energy Reviews 82 (2018): 2934-2945.
[4]: Hornborg, Alf, Gustav Cederlöf, and Andreas Roos. “Has Cuba exposed the myth of “free” solar power? Energy, space, and justice.” Environment and planning E: Nature and space 2.4 (2019): 989-1008.
[5]: Cederlof, Gustav, and Alf Hornborg. “System boundaries as epistemological and ethnographic problems: Assessing energy technology and socio-environmental impact.” Journal of Political Ecology 28.1 (2021): 111-123.
[6]: Bartie, N. J., et al. “The resources, exergetic and environmental footprint of the silicon photovoltaic circular economy: Assessment and opportunities.” Resources, Conservation and Recycling 169 (2021): 105516.
[7]: Powell, Douglas M., et al. “The capital intensity of photovoltaics manufacturing: barrier to scale and opportunity for innovation.” Energy & Environmental Science 8.12 (2015): 3395-3408.
[8]: Dehghani, Ehsan, et al. “An environmentally conscious photovoltaic supply chain network design under correlated uncertainty: A case study in Iran.” Journal of Cleaner Production 262 (2020): 121434.
[9]: Carvalho, Maria, Antoine Dechezleprêtre, and Matthieu Glachant. Understanding the dynamics of global value chains for solar photovoltaic technologies. Vol. 40. WIPO, 2017.
[10]: Dehghani, Ehsan, et al. “Resilient solar photovoltaic supply chain network design under business-as-usual and hazard uncertainties.” Computers & Chemical Engineering 111 (2018): 288-310.
[11]: Kumar, Abhishek, et al. “Economic viability analysis of silicon solar cell manufacturing: Al-BSF versus PERC.” Energy Procedia 130 (2017): 43-49.
[12]: Fritts, Charles E. “On a new form of selenium cell, and some electrical discoveries made by its use.” American Journal of Science 3.156 (1883): 465-472.
[13]: Effect of Light on Selenium During the Passage of An Electric Current*. Nature 7, 303 (1873).
[14]: Green, Martin A. “Silicon photovoltaic modules: a brief history of the first 50 years.” Progress in Photovoltaics: Research and applications 13.5 (2005): 447-455.
[15]: Perlin, John. Let it shine: the 6,000-year story of solar energy. New World Library, 2013.
[16]: Selenium Cells, Thomas William Benson, 1919.
[17]: Extrapolating from the performance of the next panel, we can guess that this one had a power output of about 25W and just under 3% efficiency.
[18]: Cove claimed to have built an even larger panel of 9 m2, but no image has survived. It was said to have had a power output of 768 watt at 8% efficiency assuming 100 W/ft2 solar insolation. This array consisted of 8 panels with a total of 14,432 plugs.
[19]: Winthrop Packard, Technical World Magazine 11, nr.4, June 1909.
[20]: Why don’t we use conductors for solar panels? When light hits a conductor surface it mostly reflects, and little or no energy is absorbed. Furthermore, in conductors, the free electrons move randomly, there is no flow of current, no directional capacity.
[21]: Cove was not the first, though. Charles Fritts’ solar cell was also based on a Schottky junction.
[22]: Byrnes, Steve. “Schottky junction solar cells.” (2008).
[23]: Tapiero, M., et al. “Preparation and characterization of Zn4Sb4.” Solar Energy Materials 12.4 (1985): 257-274. See also: Mozharivskyj, Yurij, et al. “A promising thermoelectric material: Zn4Sb3 or Zn6-δSb5. Its composition, structure, stability, and polymorphs. Structure and stability of Zn1-δSb.” Chemistry of Materials 16.8 (2004): 1580-1589.
[24]: Rothwarf, A., and K. W. Böer. “Direct conversion of solar energy through photovoltaic cells.” Progress in Solid State Chemistry 10 (1975): 71-102..
[25]: Anderson, W. A., A. E. Delahoy, and R. A. Milano. “An 8% efficient layered Schottky‐barrier solar cell.” Journal of Applied Physics 45.9 (1974): 3913-3915.
[26]: Yavuz, Serdar. Graphene/Silicon Schottky Junction Based Solar Cells. University of California, San Diego, 2018.
[27]: Todorov, Teodor K., et al. “Ultrathin high band gap solar cells with improved efficiencies from the world’s oldest photovoltaic material.” Nature communications 8.1 (2017): 1-8.
[28]: Selenium can be deposited by thermal evaporation at only 200°C. This temperature is within easy reach of solar thermal technologies, which means that in principle these processes could be run by direct use of solar energy.
[29]: Hadar, Ido, et al. “Modern processing and insights on selenium solar cells: the world’s first photovoltaic device.” Advanced Energy Materials 9.16 (2019): 1802766.
[30]: Ferhati, H., F. Djeffal, and D. Arar. “Above 14% efficiency earth-abundant selenium solar cells by introducing gold nanoparticles and Titanium sub-layer.” Optical Materials 86 (2018): 24-31.
[31]: Zhu, Menghua, Guangda Niu, and Jiang Tang. “Elemental Se: fundamentals and its optoelectronic applications.” Journal of Materials Chemistry C 7.8 (2019): 299-2206.
[32]: More details in “George Cove’s solar energy device”, Dennis Bartels, 1997.
[33]: Polozine, Alexandre, Susanna Sirotinskaya, and Lírio Schaeffer. “History of development of thermoelectric materials for electric power generation and criteria of their quality.” Materials Research 17 (2014): 1260-1267.