Continuing our series on metabolic rifts.
Nearly half a century ago, in Scientific American, ecologist C.C. Delwiche warned: “Of all man’s recent interventions in the cycles of nature the industrial fixation of nitrogen far exceeds all the others in magnitude.”[1]
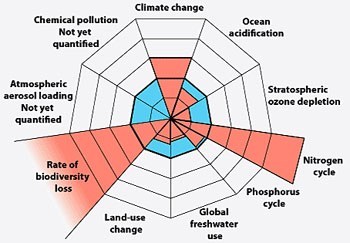
Planetary Boundaries. Nitrogen and biodiversity are farther out of safe limits than any others (Rockstrom et. al, Nature, 2009)
Although that is much more true today, nitrogen pollution is one of the least discussed environmental problems.
If you ask green activists to identify their major concerns, climate change and species extinctions will likely be named first, followed by air pollution, deforestation and maybe population growth. If nitrogen is mentioned, it will be way down the list. Although there are many scientific and technical studies on the nitrogen crisis, few popular books on environmental issues have anything substantial to say about it. Organic farmers are concerned about nitrogen in artificial fertilizers, but there are no anti-nitrogen demonstrations, no international nitrogen reduction treaties, no politicians defending or denying the science.
As the 2013 report Our Nutrient World says,
“While recent scientific and social debate about the environment has focused especially on CO2 in relation to climate change, we see that this is just one aspect of a much wider and even more complex set of changes occurring to the world’s biogeochemical cycles. In particular, it becomes increasingly clear that alteration of the world’s nitrogen and phosphorus cycles represents a major emerging challenge that has received too little attention.”[2]
The scientific case for addressing nitrogen disruption is strong. Planetary Boundaries studies have identified two critical Earth System processes that are farther out of safe limits than any others — biodiversity loss and the nitrogen cycle.[3] The journal Science describes “massive disruption of the global nitrogen regime” as a “major component” of the Anthropocene.[4] A report sponsored by the European Science Foundation says that industrial production of reactive nitrogen “represents perhaps the greatest single experiment in global geo-engineering that humans have ever made.”[5]
The rift in the nitrogen cycle is a major threat to the stability of the Earth System. This and subsequent articles will discuss how the natural cycle works and how it has been disrupted in the Anthropocene.
======
As previously discussed in this series, the growth and survival of all living organisms depends on constant recycling of matter and energy at every level, from microscopic cells to the entire planet.[6] The chemical elements that make life possible are constantly used and reused on timescales ranging from microseconds to millions of years. That’s especially true of the big four — oxygen, carbon, hydrogen, and nitrogen — that make up 96% of every human’s body.
The great biogeochemical cycles that shape and define the Earth System evolved over billions of years, taking their present forms long before our earliest primate ancestors were born. In the past two centuries, and especially since 1950, human activities have disrupted many of those metabolic cycles, undermining the systems and conditions that make life as we know it possible.
The most complex biogeochemical cycle involves nitrogen, which can be called the very stuff of life. Nitrogen comprises between 13% and 19% of all proteins, including Rubisco, the enzyme that makes photosynthesis possible. Vaclav Smil summarizes its importance:
“Nitrogen is present in every living cell; in chlorophyll whose excitation by light energizes photosynthesis (the biosphere’s most important conversion of energy); in the nucleotides of nucleic acids (DNA and RNA), which store and process all genetic information; in amino acids, which make up all proteins; and in enzymes which control the chemistry of the living world….
“It is the nutrient responsible for the vigorous vegetative growth, for the deep green of the leaves, for their large size and delayed senescence, as well as for the size and protein content of cereal grains, the staples of mankind. Nitrogen deficiency cannot be missed either: pale green or yellowing leaves, slow and stunted plant growth, low yields and depressed protein content of seeds.
“Nitrogen’s importance for human beings is no less critical. We have to ingest ten complete, preformed essential amino acids in order to synthesize our body proteins needed for tissue growth and maintenance. Stunted mental and physical development are the starkest consequences of protein malnutrition.”[7]
Of the elements that are essential for life, nitrogen is simultaneously the most abundant and the least available. There is more nitrogen in the biosphere than carbon, phosphorus, oxygen, and sulfur combined. 78% of the air we breathe is nitrogen — but over 99% of the nitrogen in the atmosphere is in a form that few living organisms can use.
Nitrogen atoms have an unusually strong ability to form stable compounds in many ways with different elements, In particular, they readily combine with various numbers of oxygen and hydrogen atoms to create ammonia, ammonium, nitric oxide, nitrite, nitrate, nitric acid, nitrous oxide and a multitude of organic molecules. In those forms, it is called reactive nitrogen because it can take part in biological and chemical processes, and because the various nitrogen compounds can and do turn into one another easily. (For plant growth, ammonium and nitrate are particularly important, and agriculturalists usually refer to those specific compounds as available nitrogen.)
But mostly, nitrogen atoms combine with each other. Pairs of nitrogen atoms bind to create dinitrogen, nearly-unbreakable molecules that are chemically and biologically inert. It is those molecules that comprise 78% of the air. The high energy and heat of lightning bolts can split dinitrogen molecules and combine them with hydrogen to create ammonia, but that doesn’t happen often enough or in great enough volumes to provide the reactive nitrogen that life needs.
(Scientific articles often abbreviate dinitrogen as N2, and reactive nitrogen as Nr. For clarity, I will use the words, not the abbreviations.)
Life as we know it is only possible because, at some time in the distant past, some bacteria evolved the ability to fix nitrogen — to split atmospheric dinitrogen molecules and create reactive nitrogen compounds. An early form of Biological Nitrogen Fixation (BNF) likely evolved over 3 billion years ago, along with the first unicellular organisms, but the modern nitrogen cycle requires oxygen, which was then rare. About 2.5 billion years ago, at roughly the same time as the Oxygen Revolution began changing the composition of Earth’s atmosphere, a few strains of bacteria and archaea, collectively known as diazotrophs, evolved the modern form of BNF. Today, descendants of those microscopic organisms are the only organisms that can fix nitrogen. No other form of life has evolved the ability, but every other form of life depends on it.
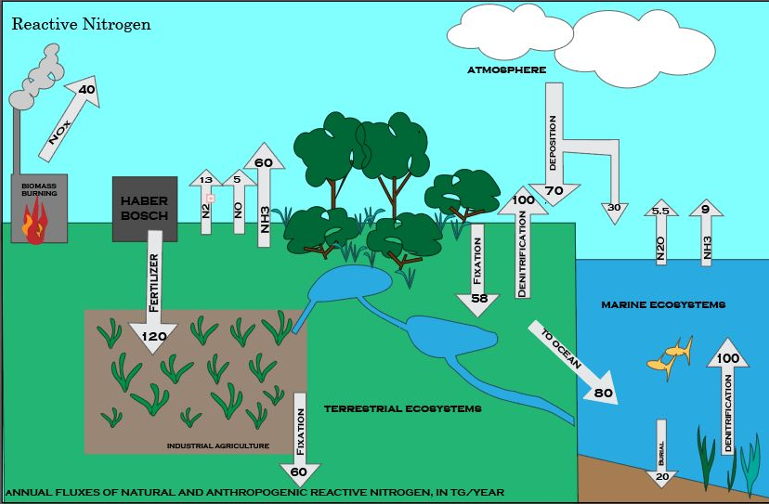
Diazotrophs and other microbes are key players in a circular process that moves nitrogen from the atmosphere to living organisms and back again. In terrestrial ecosystems, there are three major stages, each involving transformations that only microscopic organisms can perform.
Fixation. Dinitrogen diffuses from the air into soil and surface waters, where diazotrophs convert (fix) it to ammonia, a reactive nitrogen gas that dissolves in water to form ammonium. In the ocean, fixation is mostly done by some types of cyanobacteria, which are often mis-named blue-green algae. In the soil, some ammonium is created by free-living microbes, but by far the largest amount of fixation is done by a few species that live symbiotically in the roots of legumes such as alfalfa, clover and beans. Farmers discovered thousands of years ago that growing legumes along with other crops or in annual rotations helped to maintain soil fertility.
Nitrification. Some plants, such as rice, can use ammonium directly, but most do not. Specialized microbes quickly convert it first into nitrite and then into nitrate, which plants absorb it through their roots and use it to make amino acids and proteins. When plants die and decompose, the nitrogen they have used eventually returns to the soil as organic compounds that decay into ammonium, which can then be converted to nitrite and nitrate. Of course some plants are eaten by birds or animals that use some of the nitrogen to build their bodies, and excrete the rest — ultimately it returns to soil somewhere. In natural ecosystems, organic matter that has decomposed in soil is a primary source of the reactive nitrogen needed for new plant growth.
Denitrification. Some reactive nitrogen is buried in ocean sediments or deep soils, but most is consumed by other microbes that transform it into gases that return to the atmosphere. On average, the cycle from initial fixation back to the atmosphere as dinitrogen takes about 500 years for the nitrogen in soils and 10 times longer longer in the oceans.

Nitrogen cycle
This is a simplified account of a very complex process. Entire books have been written to describe the nitrogen cycle, and most admit that it is still not fully understood. Any given nitrogen atom can go through parts of the cycle many times over, in different ways and timescales, combining with other elements in multiple ways, passing through the air, water, soils, plants, animals and humans on the way from the atmosphere and back.
What’s more, the various biogeochemical cycles cannot be fully understood in isolation from each other — each one strongly affects, and is strongly influenced by, the others, as the synthesis report of the International Geosphere-Biosphere Program pointed out:
“The atmospheric concentration of CO2 can influence the amount of nitrogen taken up by plants that have nitrogen-fixing symbionts in their root structure by altering the biological nitrogen fixation rate. These particular types of plants can utilise increased availability of nitrogen to increase leaf nitrogen, which leads in turn to increased photosynthetic capacity. The biological nitrogen fixation rate, however, is also limited by another element, phosphorus. The CO2 level can also alter the amount of phosphorus available to plants.”[8]
Many more examples could be cited. If the soil contains insufficient water, plants can’t grow and take up nitrogen. If temperatures are too warm, diazotrophs can’t fix nitrogen as well. The planetary cycles are tightly interlinked, and life is an active participant.
If nitrogen fixation wasn’t followed by denitrification — if it was a one-way process and not part of a cycle — all the nitrogen in the atmosphere would have been removed long ago. But, as has often happened in the evolution of living matter, natural selection produced organisms that reversed the process. Over hundreds of millions of years, natural selection produced a balance between the conversion dinitrogen to reactive nitrogen by some bacteria and the conversion of reactive nitrogen to dinitrogen by others. As a result over time the volume of reactive nitrogen in the biosphere remained roughly constant — until recently, as we will see.
In the early 1800s, agricultural chemists enunciated the law of the minimum, which states that plant growth is limited not by the total amount of nutrients available, but by amount of the scarcest nutrient. In most cases, the limiting factor has been nitrogen in forms that plants can readily use. There is proportionately less of it in most ecosystems than of other essential nutrients, and the total amount in the biosphere doesn’t increase over time. In the oceans and on land, nitrogen is, as Australian ecologist Thomas White wrote, “the most limiting chemical.”
“As a nutrient, nitrogen is required in quantities second only to carbon. It is a key constituent of all living cells. Without nitrogen proteins cannot be built. Proteins are the basic physico-chemical structures of all living things, and are made from amino acids. Nitrogen is the key component of these amino acids which all organisms must have. No organism — plant, animal, or protist — can survive, let alone grow, without an adequate supply of nitrogen for the synthesis of proteins. The productivity of all life on Earth, in both terrestrial and aquatic environments, is limited by biologically available nitrogen.”[9]
Globally and in most ecosystems, the availability of reactive nitrogen has limited the amount of biomass on Earth, and natural selection has favored organisms that use it efficiently. But in the past century, three major processes have disrupted the balance between fixation and denitrification, by adding unprecedented amounts of reactive nitrogen to the biosphere:
- Industrial production of ammonia for fertilizers and explosives, using the Haber-Bosch process;
- Large-scale cultivation of rice, soy beans and other crops that promote production of reactive nitrogen;
- Burning fossil fuels, which, in addition to CO2, produces the gases nitrogen dioxide and nitric oxide — (NO2 and NO).
Those processes now produce more reactive nitrogen than all natural terrestrial systems combined — and there has been no corresponding increase in denitrification.
As a result, biogeochemist James Galloway writes, “we are accumulating reactive nitrogen in the environment at alarming rates, and this may prove to be as serious as putting carbon dioxide in the atmosphere.”[10]
Part two will examine the environmental impact of excess reactive nitrogen.
Notes
Many thanks to Fred Magdoff for his assistance with this article.
[1] C. C. Delwiche, “The Nitrogen Cycle,” Scientific American, September 1970, 137
[2] Mark A. Sutton et al., Our Nutrient World: The Challenge to Produce More Food and Energy with Less Pollution. (Edinburgh: Centre for Ecology and Hydrology, 2013), 1.
[3] Stockholm Resilience Centre, “Planetary Boundaries Research,”https://www.stockholmresilience.org/research/planetary-boundaries/planetary-boundaries/about-the-research/the-nine-planetary-boundaries.html.
[4] Elser, J. J. “A World Awash with Nitrogen.” Science, vol. 334, no. 6062, 2011, 1505
[5] Mark A. Sutton and Hans Van Grinsven, “European Nitrogen Assessment: Summary for Policy Makers,” 2011,http://www.nine-esf.org/files/ena_doc/ENA_pdfs/ENA_policy summary.pdf.
[6] Ian Angus, Earth’s Circular Economy: Recycling as a Law of Life, Climate & Capitalism, May 9, 2018
[7] Vaclav Smil, Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production(Cambridge, Mass: MIT, 2001), xiii-xiv
[8] Will Steffen et al., Global Change and the Earth System: A Planet under Pressure (Berlin: Springer, 2005), 29
[9] T. C. R. White, The Inadequate Environment: Nitrogen and the abundance of animals (Berlin: Springer-Verlag, 2012), 12.
Teaser photo credit: By M maraviglia – Own work, CC BY-SA 4.0